Roche/Genentech PARAISO
Why is the study needed?
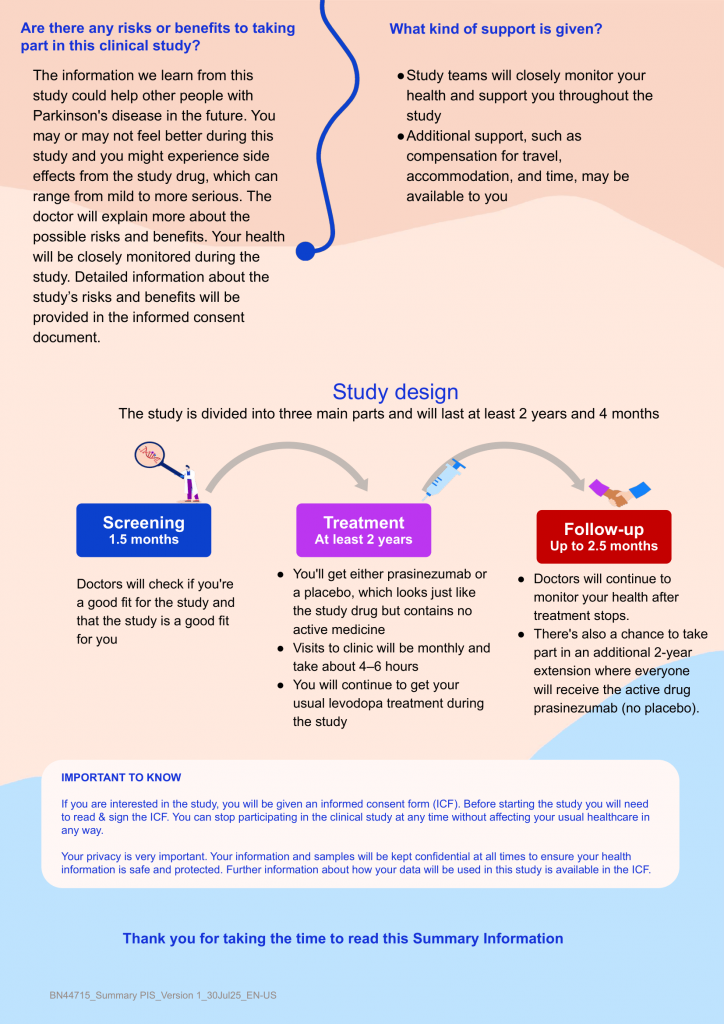
Paraiso is study to compare prasinezumab with a placebo in people with early-stage Parkinson’s disease who are taking levodopa to manage their disease symptomsParkinson’s disease (PD) is a long-term condition that gets worse over time. In PD, a naturally occurring protein called alpha-synuclein does not form properly. It sticks together to form clumps. This damages nerve cells in certain areas of the brain and causes nerve cells to die. Current treatments relieve symptoms but do not reverse, slow down or stop brain cells from dying. New medicines are needed that can prevent brain cell death to stop or slow the speed at which PD gets worse. This study is testing a medicine called prasinezumab. It is being developed to slow down the progression of PD symptoms. Prasinezumab is an experimental medicine. This means the U.S. Food and Drug Administration has not approved prasinezumab for the treatment of PD. This study aims to compare the effects of prasinezumab against non-active medicine (placebo) in people with PD who are taking levodopa to manage their symptoms.
Who can take part in the study?
-People of 50 to 85 years of age with early-stage PD (that does not affect their balance) can take part in the study if they are being treated with stable doses of levodopa for at least 3 months and have been diagnosed with PD within the last 3 years.
-People may not be able to take part in this study if they are taking other medicines for their Parkinson’s symptoms besides levodopa, have a history of other types of parkinsonian syndromes, are living in a nursing home or assisted care facility.
How does the study work?
People will be screened to check if they are able to participate in the study. This is a ‘placebo-controlled’ study. This means that participants are put in a group that will receive a medicine or in a group that will receive ‘placebo’ (a medicine that contains no active ingredients but looks the same as the study medicine). Everyone who joins this study will join 1 of 2 groups randomly (like flipping a coin) and given either Prasinezumab, given as a drip into the vein (infusion) every 4 weeks OR placebo, given as an infusion every 4 weeks. Once participants have completed the double-blinded part of the study they may choose to continue to receive treatment in an extension of the trial. Everyone who joins the extension will be given prasinezumab as an infusion every 4 weeks. The extension is open-label which means everyone involved, including the participant and the study doctor, will know the participant has been given prasinezumab. Total time of participation in the study will likely be between 2 and about 3.5 years depending on when they join. Participants have the right to stop study treatment and leave the study at any time, if they wish to do so.